
E.A.of chlorine is greater than Fluorine YouTube
Fluorine is the most electronegative element because the definition of electronegativity makes it so. The electronengativity scales are defined based on experimentally determined properties of the elements.
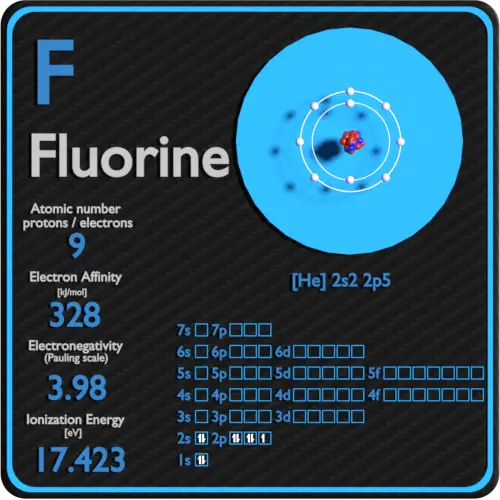
Fluorine Periodic Table and Atomic Properties
3 Answers. Sorted by: 20. Fluorine is the most electronegative element because the definition of electronegativity makes it so. The electronengativity scales are defined based on experimentally determined properties of the elements. Fluorine has appropriate values for all of the common scales to ensure it has the highest electronegativity.

Chlorine has ___________ electron affinity than fluorine. YouTube
VIDEO ANSWER: dear students in the given question we have passed to give a reason why Florence is more electro negative than chlorine. Since we know that Florina. As to shin, which is K. And L. Where chlorine is Tr

Cn Is More Electronegative Than Chlorine

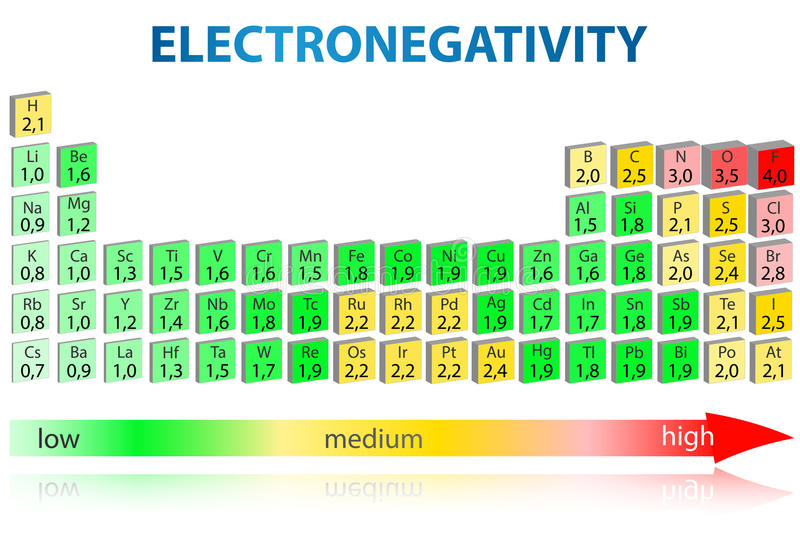
Electronegativity Chart of Elements Science Struck
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to cesium and francium which are the least electronegative at 0.7.

Polar vs. Nonpolar Bonds — Overview & Examples Expii
A qualitative answer is that fluorine is more electronegative than chlorine because the outer electrons in fluorine are less "screened" from their attraction to the positive nucleus, because there.

Fluorine Is More Acidic Than Chlorine Because Fluorine Is More
8.13.3: Chemistry of Fluorine (Z=9) Fluorine (F) is the first element in the Halogen group (group 17) in the periodic table. Its atomic number is 9 and its atomic weight is 19, and it's a gas at room temperature. It is the most electronegative element, given that it is the top element in the Halogen Group, and therefore is very reactive.

Fluorine Has Less Electron Gain Enthalpy Than Chlorine Periodic table
Why is Florine more electronegative than oxygen. Fluorine has 1 more proton than oxygen and one more electron but the same number of shells so no increased shielding. This means there is more electostatic attraction from the nucleus so increased pull from the positive nucleus means a higher electronegative value than oxygen. Answered by Jamie A.

Fluorine Electron Affinity Electronegativity Ionization Energy of
Step 1/3 1. Fluorine is more electronegative than iodine because it has a smaller atomic radius and a higher effective nuclear charge. This means that fluorine has a stronger attraction for electrons in a chemical bond than iodine does.

pls explain why fluorine has less negative electron gain enthalpy than
Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3 energy level, one would initially expect that an electron rushing towards fluorine would release more energy, as it would land in the n=2 energy level, whereas in chlorine, the electron would land only in the n=3 energy level, and would then not release as much energy.

WHY CHLORINE HAS MORE EGE THAN FLUORINE?? II EXPLAINED IN ENGLISH II
Let us first recall the term electronegativity.It represents the ability of an atom to pull electrons closer to itself in a bond. Within group, it decreases from top to bottom due to a larger distance between the nucleus and valence electrons.Through period, it increases from left to right since nonmetals have more tendency for electrons than metals.
Electronegativity Chart Periodic Table Electronegativity 397
First reason i know is hydrogen has one shell and one proton, as compared to fluorine with 2 shells and 9 protons. As electronegativity is the "strength" with which the valence electrons are attracted to the nucleus. hence fluorine have more electronegativity. . 2nd .:-.
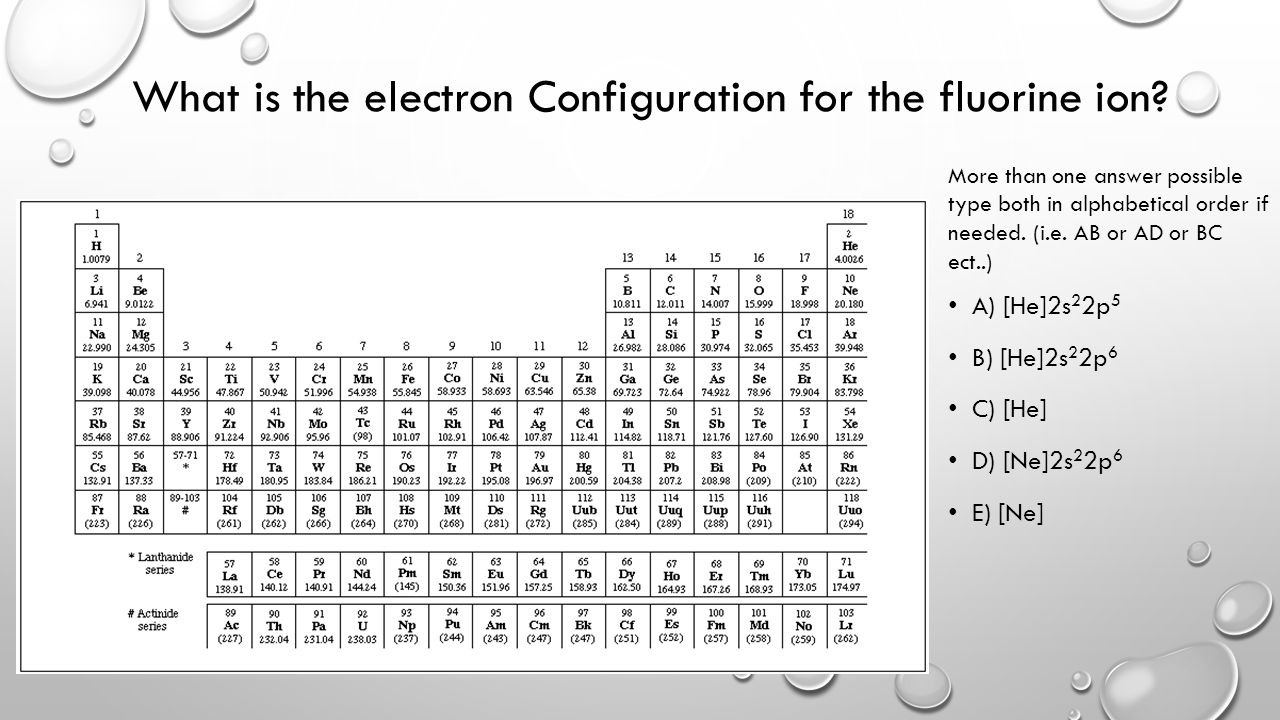
Fluorine Electron Configuration (F) with Orbital Diagram
It shouldn't really be thought of from the point of the atom, but from the point of the electron. In the case of F and Na reacting discussed in other comments, the bonding electron has a "choice", stay in Na's 3p orbital, or move into the last remaining slot in F's 2p.
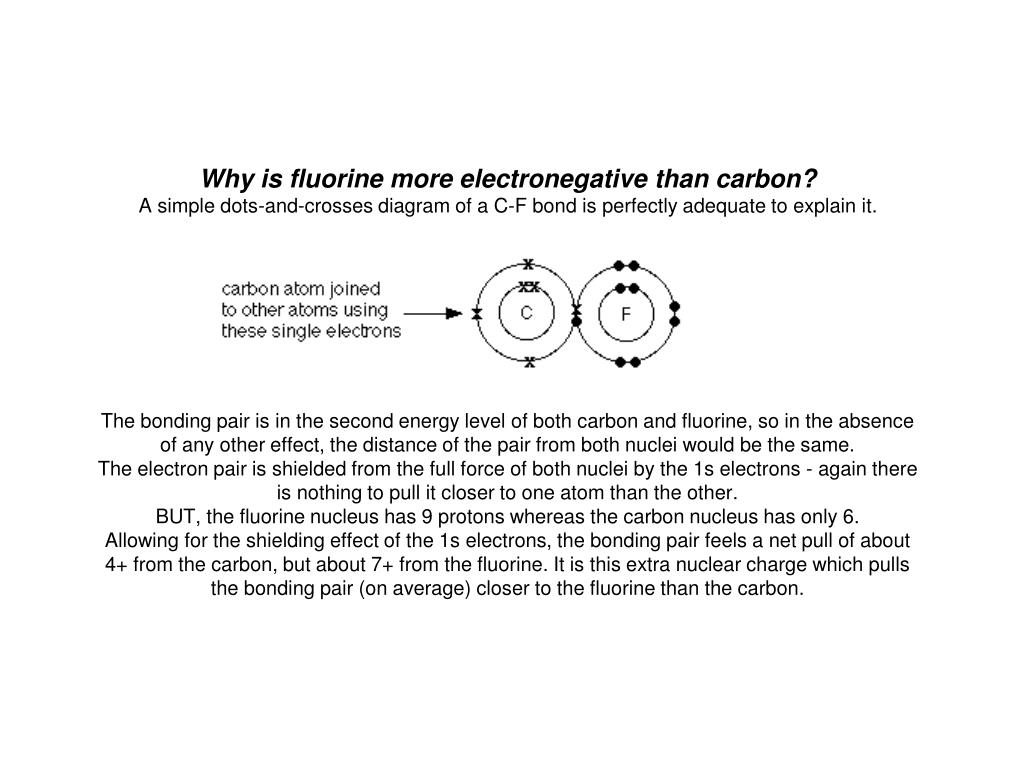
PPT Why is Fluorine more electronegative than Carbon? PowerPoint
Chemists love extremes and superlatives! We will thus give special credits to the element that comes up with (1) the smallest atomic radii of all non-hydrogen and non-noble-gas elements, (2) the largest electronegativity of all open-shell atoms (4.1 in the Allred-Rochow scale), and (3) the highest reduction potential of all elements (2.87 V).

Periodic Table With Electronegativity Values
Step 1/2 Step 1: The electronegativity of an atom is determined by the ability of its nucleus to attract electrons. In the case of fluorine and chlorine, both are in the same group of the periodic table, so they have the same number of valence electrons.

Why electronegativity of chlorine is greater than of fluorine
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is given a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7.